Improve Medical Device Design with Innovative Simulation Technology
Discover Altair Partner Alliance Technology for the Medical Industry
New Innovative Opportunities in the Compliance Assessment and Selection of Materials for the Medical Industry
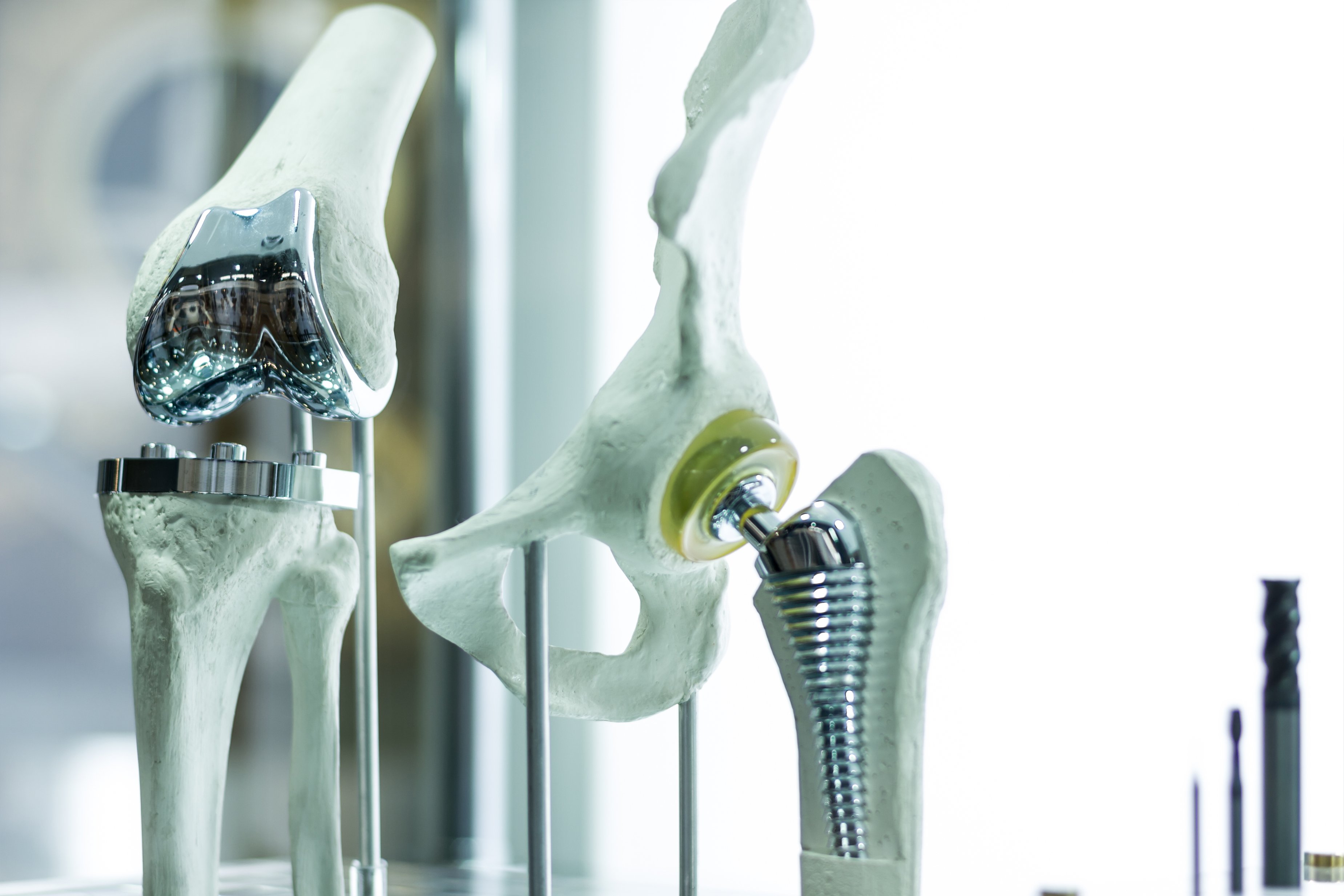
In world where the population is getting older and living longer, the need for durable medical devices and implants is exploding. This requirement drives innovation in the space, widening the scope from the traditional metals such as titanium and cobalt chromium, to newer material groups including thermoplastics and ceramics which at the same time increases the challenges in safety and the efficacy of the end product. We will cover how Total Materia can drive precise, safe decisions around material selection for a range of critical constraints, whether it be basic material durability, fatigue strength requirements or adherence to a range of regulatory guidelines including biocompatibility or compliance to other national administrative controls such as the FDA.
Presenter: Natalija Scepanovic, Applications Specialist, Key to Metals AG
Featuring Total Materia, available through the Altair Partner Alliance.
Efficient Workflow for the Simulation of Plastics in the Medical Industry
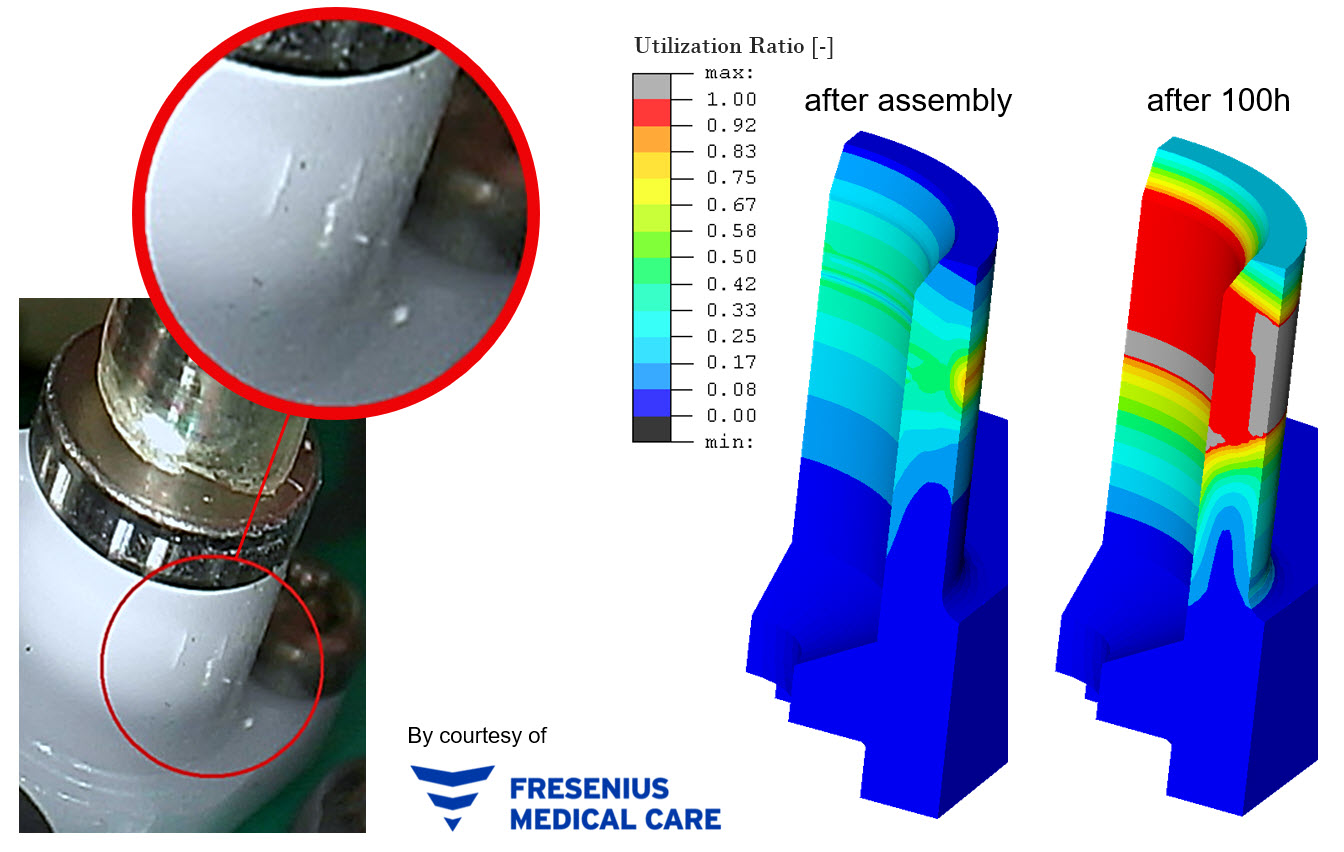
The strength assessment of plastic components is often a problem in practice. It is important for the beneficial application of simulation to achieve a good trade-off between the simplest possible modeling approaches (material model, strength assessment) and sufficient fidelity of the simulation results. If non-standardized simulation and assessment approaches are used, or experience-based, subjective procedures are employed, uncertainties follow and the comparability of different analyses is often not possible. Therefore, a workflow of Altair and partner software is shown to avoid such uncertainties and two examples from medical industry are used to display the benefits. Simplified procedures help to reduce the work load and increases the confidence in simulation results. The standardized procedure avoids mistakes and assures that reproducibility and comparability of the results is always given.
Presenter: Sascha Pazour, Engineer, PART Engineering GmbH
Featuring S-Life Plastics, available through the Altair Partner Alliance
Optical Modeling of Medical and Biomedical Systems Using TracePro

The design of medical and biomedical systems can present unique and challenging obstacles. Factors such as scattering from tissue, high absorption, and low signal levels need to be addressed. Optical design and analysis programs such as TracePro feature tools such as bulk scattering and importance sampling that can help with this. TracePro allows the user to design, optimize, and verify the system in a virtual environment, decreasing development time and cost as well as reducing time to market. Designing with TracePro allows the user to try a large number of variations in a short amount of time and sometimes find solutions that may not have been considered initially. In this webinar we will look at several types of systems in TracePro including pulse oximetry and fluorescence microscopy.
Presenter: Dave Jacobsen, Sr. Application Engineer, Lambda Research Corporation
Featuring TracePro, available through the Altair Partner Alliance




